Articles
Pandemic Pathogens
Pandemic Pathogens
A sufficiently bad disease could have disastrous effects for humanity, like the 1918 flu, black plague, or even worse. But to protect ourselves as a species from global catastrophic biological risks (GCBRs), we need to know which features make a pathogen more or less likely to pose an existential threat. In this advanced talk from EA Global 2018: San Francisco, Dr. Amesh Adalja covers what types of pathogens are most likely, which transmission methods to worry about the most, and many more considerations for anticipating and preventing GCBRs. A transcript of Amesh's talk is below, including questions from the audience.
The Talk
I'm going to talk about the characteristics and traits of pandemic pathogens. You just heard my colleague Crystal's talk where she introduced the concept of Global Catastrophic Biological Risk (GCBR). I'm going to try and delve very deep into that concept, to try to understand what is it about certain pathogens that allows them to cause a GCBR. I think the theme of this conference is to be curious, and that's really what motivated this project, was to be very curious.
I have a picture of my blog there, Tracking Zebra. If you're interested in infectious disease, I do write a lot about that, and that's my Twitter handle.
The aim of the project I'll discuss today was to develop a whole new framework around the traits of naturally occurring pandemic pathogens that could constitute a GCBR, in order to change preparedness activities. In the past we really focused on list-based approaches, that are largely derived from the former Soviet Union's biological weapons program. There hadn't been a lot of fresh thinking. It was very static. In this project, we tried to bring more fresh thinking. First I'll provide a couple of basic definitions, just because I don't know if everybody has a biological background.
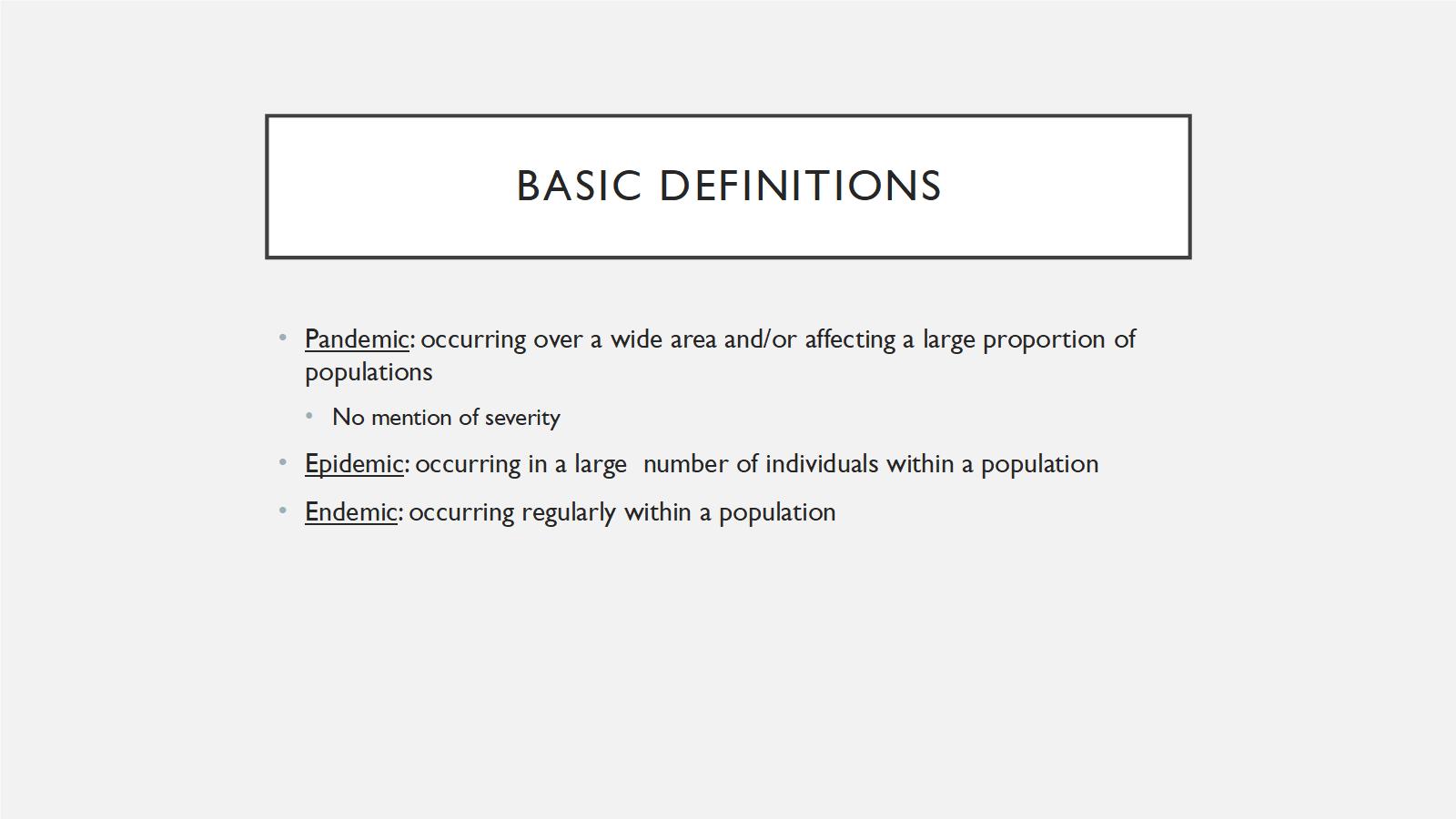
Pandemics are infectious disease outbreaks that occur over a wide area and affect a large proportion of the population. They don't necessarily have to be severe; 2009 H1N1 was a mild pandemic, but it was still a pandemic. An epidemic is an infectious disease outbreak that occurs over a large number of individuals within a population. So, the SARS outbreak in 2003 would be an epidemic. An endemic is something that occurs regularly within a population. For example, the common cold is endemic in the human population. Those are just things to keep in mind. What we're talking about are specific types of pandemics that are very severe, to meet GCBR criteria.

I'm not going to spend much time on this definition because Crystal really went into some great detail, but what I'm going to do is expand on what's special about certain biological agents, and what types of biological agents can cause GCBRs. Everybody is very focused on viruses, some people are focused on bacteria, some people on other things, so what I'm trying to understand is what traits does a biological agent have to have in order to be able to cause something this severe, to cause this massive catastrophic loss of life, disruption of society, etc.
I just want to draw a distinction, because what I'm talking about here are pathogens of pandemic potential, versus just critically regionally important, a distinction made in Mike Osterholm's recent book. When you have an outbreak like Middle East Respiratory Syndrome, just because it's not a GCBR doesn't mean it's not important or that it's not going to be very disruptive to people's lives, to societies and to governments. But what we're talking about in a GCBR is something that's going to be global, like the 1918 flu. Something that's a lot different in scale than even Middle East Respiratory Syndrome or SARS. Something much different. There's lots of pathogens of critical regional importance. Even the Ebola outbreak in 2014 in West Africa falls under the criteria for critical regional importance, versus GCBR.
The specific aim with this project was really to, like I said, move away from a list-based historical approach. People had really just taken the Soviet Union Biological Weapons program and added a couple things here and there, but really hadn't thought much about why each thing was on there. They hadn't really challenged the assumptions that put them on there, and really try to understand certain questions. What it was that made smallpox so scary? What is it that makes pandemic flu so scary? We're really trying to go into an inductive manner, trying to make a whole new paradigm, looking at the actual attributes of GCBRs. We tried to do this by being totally microbignostic. What I say microbignostic, that means we didn't go into this project saying, "This has to be a virus. This has to be a bacterium". We said it could be anything. It could be a parasite, a protozoa, it could be a prion. So that was something that was totally new. We really wanted to challenge thinking and then take this paradigm, and hopefully use it to move forward when we think about preparedness and try and think of new infectious disease outbreaks with this new paradigm in mind, to get better at being prepared because we're constantly surprised, which I'll get to later in the talk.
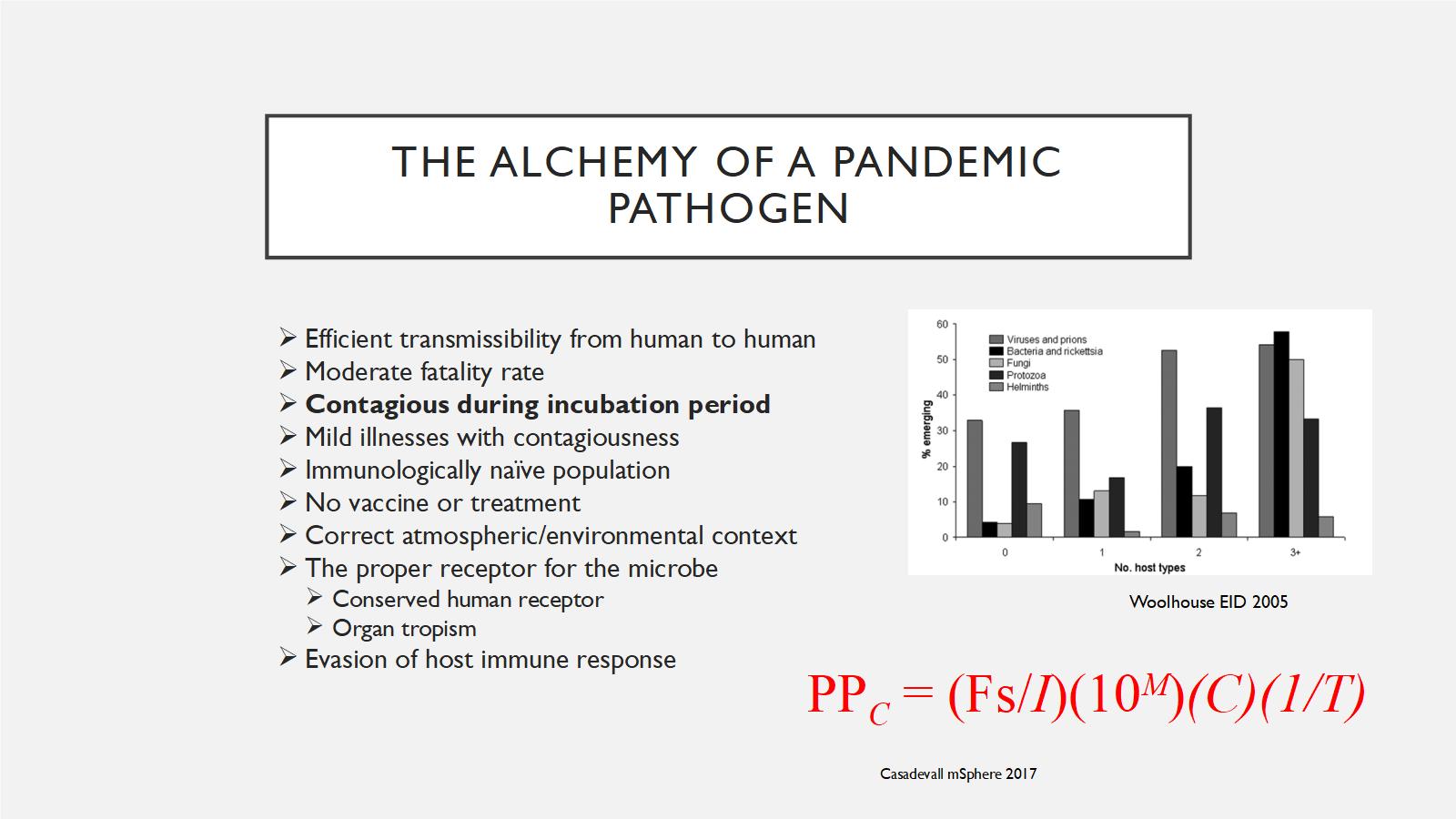
What are the essential traits? The next slide is a little busy, so I'm going to walk through it one by one. We think about what a GCBR have to have. We talk to people and do a lot of literature review; there's a whole bunch of different things that make up the alchemy of a pandemic pathogen. I'm going try and explain what this equation means as best I can.
The first thing you need to do, is you have to have a pathogen that can efficiently transmit from human to human. You can have a disease that can be really bad, like tetanus. But since tetanus doesn't transmit between humans, so it can't be a pandemic pathogen. When you're talking about a pandemic pathogen, it has to be able to get from people to people, so that's number one.
It has to have a moderate fatality rate. It doesn't have to be really, really horrible like a 90% fatality rate or 100% like rabies. It has to be something that's kind of in a sweet spot that it allows enough death to occur that it causes disruption in the society. Remember that the 1918 influenza pandemic, which killed 50 to 100 million people, only had a fatality rate of two percent. But because it was so widespread, it led to disruption.
Contagious during incubation period. I have bolded this because in multiple modeling studies, and in experience - and Crystal alluded to this earlier when she talked about smallpox - if a disease is contagious during the incubation period, when you're not sick, then it's very, very hard to control. That's why the H1N1 pandemic had spread everywhere before anybody even knew about it because people were contagious one day before symptoms. If a disease is contagious in that period, it becomes very, very difficult for public health interventions to have any impact. The same goes with mild illnesses with contagiousness. When you have the flu or the common cold and you're out shopping, doing your normal daily life, you spread that to other people. It's very hard to stop that, versus something like Ebola, when you're sick and highly contagious you are in bed and you can't really move, and move about in society. So this is another key factor.
An immunologically naïve population. Again, reflecting back on Crystal's talk when she showed the map of the indigenous populations in the Americas, in the pre-Columbian area in 1492. That was an immunologically naïve population to smallpox, which allowed smallpox to spread very rapidly through that population. That's what a pandemic pathogen would require.
No vaccine or treatment. You don't have any way to stop this. That's another thing that fits into GCBRs.
Correct atmospheric and environmental context. Infectious diseases happen in a context. Is it happening during World War I like the pandemic flu did in 1918? Is it happening where there's been societal disruption? Like, for example, Yemen right now and the cholera outbreaks? All of that's going to play a major role in how prolific an infectious disease outbreak is.
There's a lot of biology that has to go into this too. Not every pathogen can infect every type of human. You have to have a proper receptor. So you've got to have some receptor that lots of humans have that this virus or this bacteria can actually cling onto. It's also going to explain which organs it affects, because obviously some organs are more important. If it affects your brain, your kidney, your liver, your lungs, those are what you really see with these pandemic pathogens. And then it has to be able to evade the host immune response. It has to be something that is not easy for the immune system to mount an effective response against.
This is a fancy equation that showed up. The point of this equation is not to memorize it or to think about it, it's just that you can take all of these things and give them values, and come up with the pandemic potential of a pathogen. You can look and vary them. You can look at some other things too. For example, the more host types a pathogen has, the more chance it is to emerge. That's another thing that comes up, that these things can infect more than one type of species. That's where the concept of zoonosis comes about, where something comes from an animal species into humans. But the more hosts something has, the more likely it is to be able to infect humans and cause a problem.

When you think deeper, there's a couple of things that come out of this. When you look at the way these things transmit, the most likely way to cause a pandemic is for it to be done through the respiratory or the airborne route. There's much, much less you can do to stop an airborne virus or a respiratory/droplet spread virus. If I had measles right now, you would all be exposed. It's very, very hard to do that. But if it was something that was spread through, for example, fecal/oral, like hepatitis A or cholera, you can really delimit that with sanitation. Remember, there was a couple of cases of cholera in Mexico about five years ago, and everybody panicked. But there was just even a modicum of sanitation, and that stopped cholera. It did not spread in Mexico. You can't do that with respiratory viruses and airborne viruses. It's much, much harder through the respiratory or airborne bacteria.
A vector borne transmission, so that means through mosquitoes, through ticks, that's something that's very interesting and it is something that I think we struggle with trying to figure out exactly how bad a vector borne outbreak can get. They are, in general, limited by the vector range. For example, mosquitoes frolic in places that are going to be much more conducive to their habitat, so it's going to be very hard for them to live in a temperate climate. I live in Pennsylvania; we don't have mosquitoes year round there. But there are parts of the world that mosquitoes thrive in all year round. That's why I talked about specifically aedes mosquitoes, which are the mosquito that's responsible for spreading dengue, chikungunya, zika, yellow fever. They are basically covering half the population of the globe. That is something that's a little bit different with vector borne. In general, we don't think vector borne could do this because the host range, the range of the vector, is kind of limited.

The other thing to think about is, talking about global catastrophic biological risk, we talk about deaths, but is fatality everything? This is a question to pose to yourself. There's two purposes for an organism. To survive and to reproduce. What if you had a disease like zika, but which was much more widespread? Or rubella, pre-vaccine. If you decrease the reproductive fitness of a species, could that lead to a GCBR? I think the answer is yes. I don't think that zika or rubella really meet those criteria, and maybe if we were talking about this, if 4ubella occurred now, maybe it would fit in the GCBR. Back in the 1960s, people coped with rubella, but it is one way to think about a GCBR that doesn't end up killing everybody.
The other thing is, there's another virus, HTLV, which as been in the headlines a lot in the last couple of weeks because of some studies that have come out of Australia. HTLV is the most carcinogenic virus. It causes human t-cell leukemia. This was the first human retrovirus that was discovered. What if an infectious agent causes cancer in everybody? Would that lead to a GCBR? That's something just to think about, that it's not always going to necessarily be death. I think you have to be very broad and active minded about this.
There's also something I wanted to talk about called sapronotic disease. That's a term that we found from the plant world and the animal world, where you have this idea that if an infectious agent is killing everybody that it infects, it's not very good because it's going to run out of people to infect. But what if it doesn't care? What if people are just a secondary source for it? What if it's an amoeba that eats things in pond scum, but only intermittently infects humans, like the brain-eating amoebas that always grab headlines? How does that fit into this? If it doesn't necessarily need to be in humans to thrive. That it has other things. It could eat dead bark on a tree, or it can eat stuff on the bottom of the forest floor. That's another way to think about a pandemic pathogen, that I think is really interesting.
The first set of conclusions we drew from this project were, the traits that are most likely to be possessed are going to be respiratory/droplet transmission, with fecal/oral much less likely. The aedes vector borne agents, those mosquitoes, have a special status because there's widespread mosquito prevalence and there are certain viruses that get very high levels in your blood that these mosquitoes can just kind of pick off. That's why we've seen explosive outbreaks of dengue, chikungunya, zika, why we've seen yellow fever resurface. So they are a special category. They probably don't fall as high as respiratory/droplet, but that's something to keep in mind, that these could possibly do that.
So then the next thing we try to do is think about, okay, we've said respiratory/droplet transmission. So we have to make a choice, we have to think: is it gonna be virus, bacteria, fungi, parasite?
So I say there's no agnostics in a foxhole. We had to push people that we talked to in this thing, and push ourselves to think, what would it really be? So I think that viruses are very formidable in this realm. They mutate much, much more rapidly than bacteria. The transmission and replication cycle in a human is much faster than in bacteria. And you heard this earlier today, that there's no real broad spectrum antiviral agent.
I have a picture there, this is from Epcot Center, for penicillin. So when we think about bacterial infections, we can usually, even in the face of antibiotic resistance, craft together some regimen that works. And lots of them are nonspecific, they kill wide varieties of bacteria. They have a spectrum.
We don't have that so much with antiviral agents. We've got a certain drug for flu, a certain drug for hepatitis C, a certain drug for HIV. We don't have broad spectrum antivirals. And that's a major chink in the armor against viruses.
When you think about bacteria, they really are limited. Because of broad spectrum antibiotics, they're slower, they're less mutable. There are some caveats. We do have multi-drug resistant bacteria that can be challenging, and antibiotic resistance is probably one of the most pressing public health threats that we face.
But they still don't rise to the fact of causing a GCBR. Because even if you think about everything becoming resistant, we would be pulled back to the pre-penicillin era. But people still lived during the pre-penicillin era, in a way that they were still flourishing, human populations were still growing pre-penicillin. So I think that's important to remember.
But we have seen a major outbreak. For example, we talked about Yemen and the cholera outbreak that's occurring with the bombing that's going on, and the infrastructure problems, it's been the worst cholera outbreak in history. And we had a bad plague outbreak just a couple of years ago in Madagascar, with 1200 people infected.
So in certain resource-poor areas, you can see bacteria get very close to causing a GCBR. But it would be hard for it to do anything. So you think back to the Black Death back in the 1300s and 1400s. There were no antibiotics and I think that's why the Black Death probably does qualify for a GCBR at that time.
So the other thing, what about fungi? Fungi are everywhere in the environment. But the fact is, they're temperature restricted. They don't like to grow at human temperatures. And there's a lot of hypotheses about why this is. They think that humans actually came through what's called a mammalian filter, that we evolved the ability to have a 98.6 degree Fahrenheit or a 37.5 degree Celsius temperature as a way to avoid fungi. And we see fungi decimating salamanders and frogs, and all of these other types of species that have lower body temperatures.
And it's very, very hard for fungi to infect humans, they usually have to be immuno-compromised. There's been a few scary things, like candida orus, which is a multi-drug resistant fungi that preys on hospitalized patients. Cryptococcus gattii, which is one that kind of is around the soil and trees. And then there was this exserohilum outbreak. That was related to contaminated steroids, which you might've heard about that getting injected directly into people's backs. But again, they needed something to be able to do that.
Some of these fungi are sapronitic and they can be very, very high mortality rate because they don't transmit well between humans. But the temperature restriction makes it very hard for them to cause a pandemic.

For prions, you've probably heard of mad cow disease, which is probably the most famous prion disease. Those don't spread very well between humans, unless there's cannibalism. This is a graffiti in Pittsburgh, there's a guy who writes kuru all over the city of Pittsburgh. Kuru was a disease that was found in Papua New Guinea among the indigenous tribes there, where they were engaging in ritualistic cannibalism of each other after someone died, and kuru was really decimating that population.
But unless you have people directly exposing themselves like that, in that manner, prions are unlikely to do that. We've seen scrapie for example, in the sheep species, and then chronic wasting disease in elk. Those things have done - because there's so much different saliva contact between people and the way these animals eat and interact with each other, that don't really apply to humans.
It's interesting, because when you think about prions, chronic wasting disease, they actually had to burn down a forest to stop it from spreading, because it was so endemic to the elk and deer in that region. So that's a testament to what a prion can do, but it's very restricted when you think about how prions could do this in humans.

This is something that most people don't know about. So we look to try to see, was there ever an extinction event from an infectious disease in any animal species? And there's one. This is the Christmas Island Rat, and it basically was driven to extinction, by a vector-borne, so mosquito-born, trypanosome. Trypanosomes are parasites, much bigger than bacteria or viruses. And the Christmas Island rat basically was rendered extinct because of it.
But does this apply to humans? I don't think it does, because the poor Christmas Rat couldn't go anywhere. It was on an island; there was nowhere for it to run. Humans can outrun a vector, and like I said, vectors are limited in where they can actually cause disease and where they can spread infections. And if you can outrun that vector, if you can move to places where it isn't there, you can probably avoid it.
I think that when you look at the human trypanosome diseases, they haven't done anything near what they did to the Christmas Island rat. There are some concerns when you talk about parasites and protozoa, about certain drug-resistant forms of malaria, especially if they make it from Asia to Africa, being able to maybe cause something on a GCBR level. But we haven't seen that yet.
There's lots of other things to think about when you think about infectious disease. Helminths, ectoparasites, amoeba, non-carbon based, so that's something when you talk about the mission to Mars, and thinking about how you're going to deal with organisms that may or may not come back there.
So there's lots of different things to think about, but really our consensus was that viruses are most likely going to be the GCBR agent, because of their mutability and their rapidity of spread, and their lack of an antiviral.
But when you get into viruses, there's so many viruses. There's lots of different rules and exceptions. Would the virus's genes be RNA, or DNA? Will it copy itself in the cytoplasm of a cell or will it be in the nucleus? Will it have a segmented genome like flu? Flu is one of the most prolific viruses, and the reason why it's so good at infecting people is because it can shuffle its genes, because they're all on a segment, and it can basically be like a deck of cards that switches different genes. So that's something that is really important to think about, whether it's segmented versus non-segmented. Does it have a really big genome like MERS and SARS? Or is it a very small genome?
And then what about these ones that are spread by mosquitoes? They get very high levels of the blood in people, are those the kind of viruses that are gonna be able to cause GCBR? In just a couple of headlines a monkey pox, which is a DNA virus that people are very nervous about in the wake of the smallpox eradication, people aren't vaccinating for smallpox routinely anymore, now monkey pox has resurged and the vaccine was protective against monkey pox.
So when you think about viruses, there's lots of different things to think about. Probably when you think about GCBR-level risks, flu goes to the top of this list, because it's done it so many times before. And we do have this scare right now that's been going on since probably the 1990s, regarding avian influenza. That's a picture from my local county fair in my hometown outside of Pittsburgh.

And when you think of avian flu, one of the scariest versions of this is H7N9. We're right now in the sixth wave, but in the fifth wave we saw some very, very scary things happen. You saw changes showing this virus being more likely to be able to transmit between humans, we've seen antiviral resistance in this strain, we've seen the genetics of the strain change so much that the vaccine that's stockpiled, there was a mismatch. And we've seen it evolve high pathogenicity in chickens.
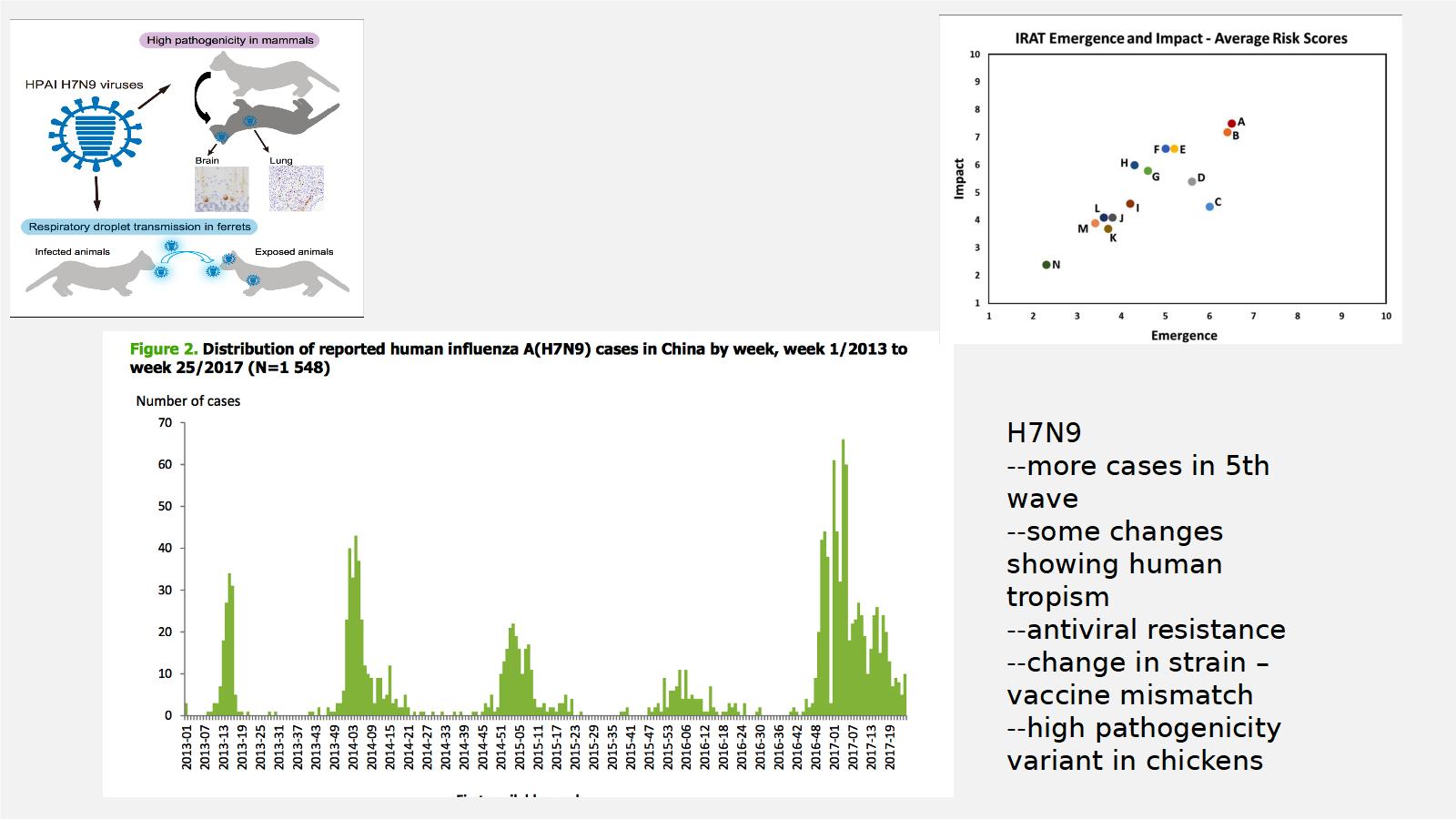
This is the CDC's ranking of viruses. A and B are both H7N9. So it's at the highest risk for emergency impact. So this is probably one of the scariest viruses that we face. And it meets a lot of those criteria that we talked about.
When you look at the steps in pandemic emergence, there's a bunch of steps that a virus has to do, this is specific for influenza. We're already down to around 3 to 4. The infection is replicating sufficiently to produce infectious virus, but we're seeing very stuttered human transmission. But we're getting down that road with H7N9.
So this isn't something that's very theoretical, what I'm talking about, this is something that we're dealing with today, now, in China, with H7N9.
I think what the CDC does is they actually rank the different properties of the virus, which I think is a very good thing to do. It might not always be accurate, but it does definitely give you some framework for how to evaluate viruses, and that's what we were doing with this framework, was trying to take this type of an assessment tool of viruses, of influenza, and apply it to the whole microbial world. And that's what this new paradigm that we made was going to try to accomplish.
So what do you do when you come up with these ideas, when you come up with these types of lists of things that might cause this? You can do what CEPI did. CEPI is the Coalition for Epidemic Preparedness and Innovation. It's a major funder for vaccine research, and what they did is they came up with a list that they took from the WHO blueprint for research, and picked some to actually go after.
I think that's one way to do this, where you have diseases that meet this criteria, then you invest money to go into it, to develop vaccines.
A couple of other things, just a couple other headlines just to show you. People did think about space bacteria, but it's unlikely that space bacteria, if they're adapted to Mars, are going to be able to do very well in humans on Earth, because there's totally different conditions that would allow them to flourish on one planet, that wouldn't apply to another planet.
We talked to lots of people that were thinking about salamanders and frogs, which are being decimated by these sapronotic pathogens, but don't necessarily affect humans.
So a couple more conclusions there. Any microbe is capable of causing a GCBR. But we believe that RNA viruses are the most pressing and likely threat, because of their mutability, their zoonotic potential. Bacterial antimicrobial resistance is unlikely to reach GCBR levels, and GCBR level of widespread fungal disease is unlikely due to its temperature restrictions, and there are very select conditions for a prion-caused GCBR.
The last part of the talk, I just want to emphasize a few things. We're always surprised about infectious disease, and I list a bunch of them here. H1N1 coming from Mexico, zika, SARS, MERS, and there's lots of people investing in surveillance and prediction approaches.
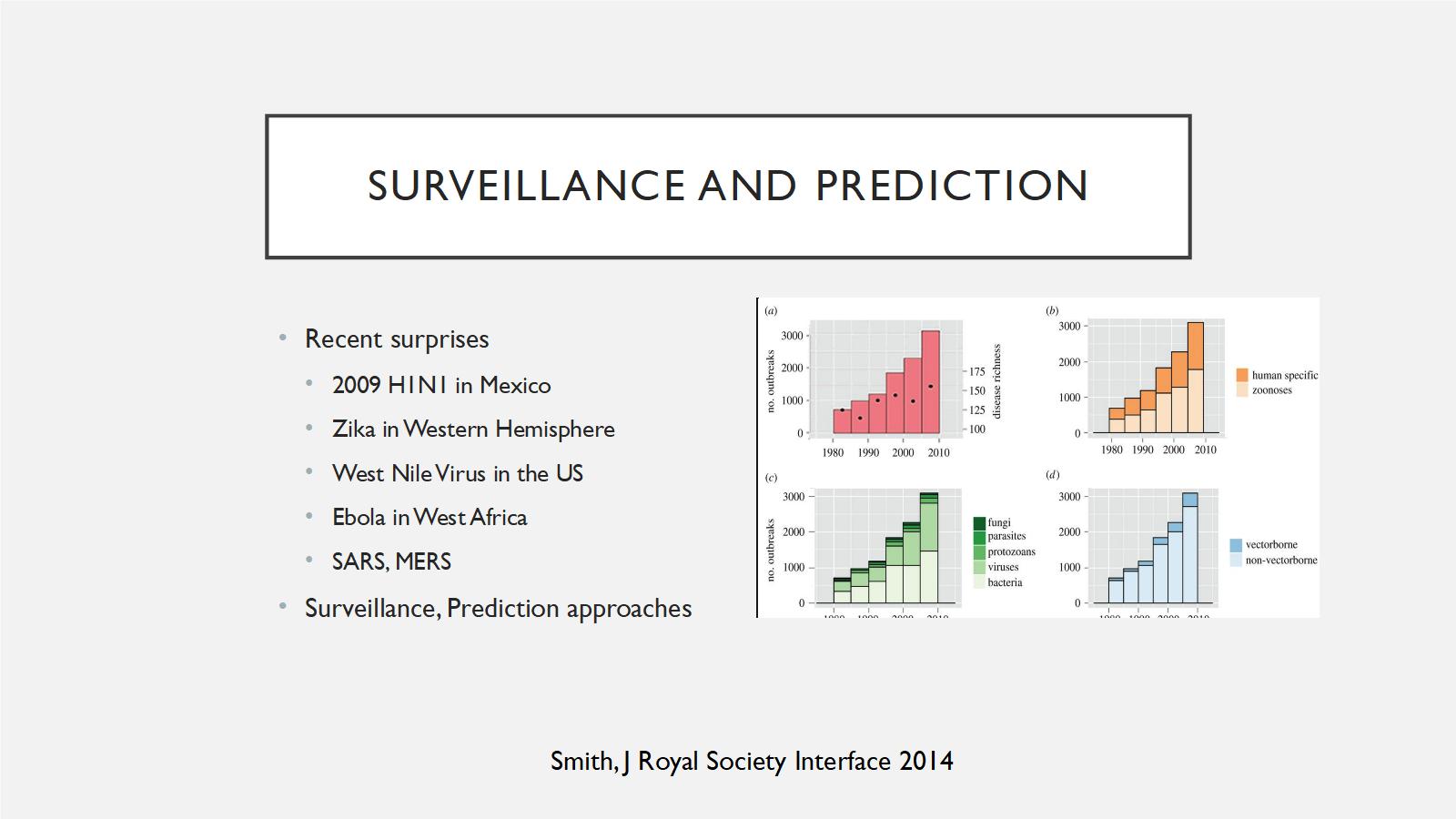
I think there's two basic approaches. There is this global virome approach, where people will go out and sequence everything that they want to do, and try to find a list of viruses that are out there. It tells you maybe what's coming, but it's very expensive, and 99 percent of those viruses are probably not going to pose any threat to humans. Also, what if the next GCBR or the next pandemic is not viral?
So that's one way to do it. Or there's another way to do it. I think this is the way I favor. It's looking at people that are getting infected by novel diseases. Looking at people like bush meat hunters, people who work in abattoirs, looking for what's called viral chatter, things that go from the first forays of a pathogen into humans. And then, looking at different hot spots. Instead of trying to sequence things, focus on things that are actually causing infections.
And then you think about unknown diagnoses. 50% of our septic shock cases, even in the United States, don't have a diagnosis, and I think that people just treat for symptoms and not necessarily for fevers. So I think that we're going about this a little bit wrongly. I think going after these unknown etiologies, all over the world, trying to figure out where these infections are occurring and actually running things down to specific diagnoses, instead of just saying, "You've got some viral syndrome." I think that's the way to go about it.

A headline just came out of Nature a couple of days ago that really validates what we're saying, which is that you should spend much more on surveying actual infections, not on trying to predict things by viral cataloging. So, what's out there, is lots of biological dark matter. We've got lots of viruses out there, lots of bacteria out there that nobody knows about. And I think we're at the stage now, that we have this tricorder culture, that Captain Kirk is holding there from Star Trek, where Bones, the doctor, would just scan someone and know exactly what they have.

We've got lots of new technologies, and I think that you can start and figure out what disease X is going to be, and that's the new WHO nomenclature for the unknown unknown. And I do think we're at that point now, but only if we actually harness these diagnostic tests.
When you think about infectious disease risk, you have to also think about human actions and that there are human actions that can enhance pandemic potential. Mistakes, political or scientific, fears of the unknown and also, complex disasters. So, if there's a war, for example, at the same time as an infectious disease, it can raise something to a GCBR-level.

So I'm going to conclude here, people keep asking me for books to read. There are lots of people who are first trying to get into this field. These are some of the ones that I could think of, that I think are interesting. This is an article I wrote for The Atlantic, Why Hasn't Disease Wiped Out the Human Race? If you just Google that, you'll find that under my name in the Atlantic, where I try to summarize some of the stuff that I talked about here. And then, these are some interesting books that I think really give you a flavor for this, and a few other ones that I didn't have pictures of.

A Viral Storm by Nathan Wolf and Level 4: Virus Hunters of the CDC by McCormick, that's the book that really got me interested in all of that. It came out in 1996. And this is the book that really started for me when I was a little child. That was the one that my parents read me over and over and over again, which is the story of the rabies vaccine. So, thank you for your interest and I'm happy to take any questions in the time that's remaining, and I have office hours as well and feel free to follow me on Twitter or read my blog. Thanks again for your attention.
Questions
Question: What are the challenges in creating a broad-spectrum antiviral? You alluded to it a bit, but, tell us more.
Amesh: In general, antiviral therapy has lagged behind antibacterial therapy by a long shot. We haven't had many antivirals ever, until actually the modern era with HIV and hepatitis C. Viruses are much trickier to make an antiviral agent against, because remember that viruses don't have their own machinery, they're inside a human cell, so they're going to be using your ribosomes, all the stuff that you use to make protein. So, many of these things can be very toxic because they're going to be hitting things that your cells do as well. So you have to find something on the virus that only affects the virus and minimally impacts your own cellular function.
So that's very hard. Whereas, in a bacteria or a fungi, they're doing their own thing, so you can find things that target just them. With antivirals, it's very hard, you have to make sure, the toxicity would be too high if it's actually blocking your proteins' synthesis and not just the virus's. So I think that's the biggest challenge, is the fact that viruses use your stuff to actually do their functions and you can't explicitly target a virus the same way you can target a bacteria or a fungi or a worm or whatever else it could be.
Question: How does monoculture kind of fit into this? I mean, farming of animals mostly, maybe other things that humans cultivate as well that kind of become global. Does that increase our risk in a material way?
Amesh: So that was something that we thought, with the monoculture you're thinking about, we were thinking mostly about humans here, so are humans a monoculture? And I don't think that that's the case, because it's said that the human immune system, within all the different humans that live on earth, can actually respond to any type of antigen. There's been some papers written about this, that the diversity of the human immune system globally, is enough that there's nothing that could really drive humans to complete extinction on its own. But, when you think about monoculture for other things, for animals or for plants, that clearly makes them vulnerable to a pathogen that could wipe them out, but not so much for humans. I think that there's enough genetic diversity, that we would be able to survive, at least a good proportion of us, with an infectious disease outbreak.
Question: How about things that may be dormant for a long time or asymptomatic for a long time, how does that kind of change the analysis of this stuff?
Amesh: That's an interesting question. So when we talked about GCBR and the definition that we use to bound this, we talked about sudden, the word sudden. And when you think about a disease like HIV, what gave HIV the ability to cause this global pandemic like no other, basically, was the fact that it has this long latency period where people were still infectious, for 10 years about, from infection to when they would start showing symptoms, and in all that time they were contagious. So, that was very advantageous for HIV. When you're talking about GCBRs, then we're talking about the speed of it, but I do think that when you talk about this long latency and dormant period where someone can transmit a disease, that's a very, very huge advantage that a pathogen would have, and able to disseminate through a population.
It's unclear whether that would qualify as a GCBR because there would be time to prepare there, there would be people looking at it the way HIV is. And I think we struggled a lot with our definition of GCBR because HIV meets a lot of these criteria, but in terms of its rapidity, it's much different than the black death or 1918 flu.
Question: About synthetic biology and the apparently ever-lowering barrier to entry into synthetic biology with, recently, hacker spaces for biology: How much does that worry you and how much should it worry me?
Amesh: So, synthetic biology, the democratization of biology and the biohacker culture, are kind of a two edged sword. You get a lot of great minds now being engaged in awesome research and that could probably lead to new treatments, new cures, new discoveries, new types of knowledge, and I think that's a great thing. But the issue, I guess, is that there are people that would try to use this for nefarious purposes and I think that there's a fine line that has to be walked there. And I would say that, although it's becoming very, very simple to do synthetic biology, it's not easy to do. It's still not something that someone can do in their back yard or without any kind of proper training. So, I do think that, some of that risk is a little bit misplaced, because I don't think that people can just concoct these chimeric, crazy things in their garage and release them.
But I do think that it's important that they start to respect norms of biosafety and understand that when you work with these pathogens, even if you're not going to create the Andromeda strain, you need to be careful and actually understand that there is a science of biosafety and it's really important that you actually follow that so that you're not going to expose yourself or others to risk. So I think that this is something that people have been engaging with this community to try and teach them this type of stuff, but I do think they should be able to flourish. I think it's really great that they're able to do this type of work without the confines of a traditional academic career.